Patients with brain cancer may benefit from treatment to boost white blood cells
Blocking immune suppressor cells in mice with glioblastoma improved survival
 Getty Images
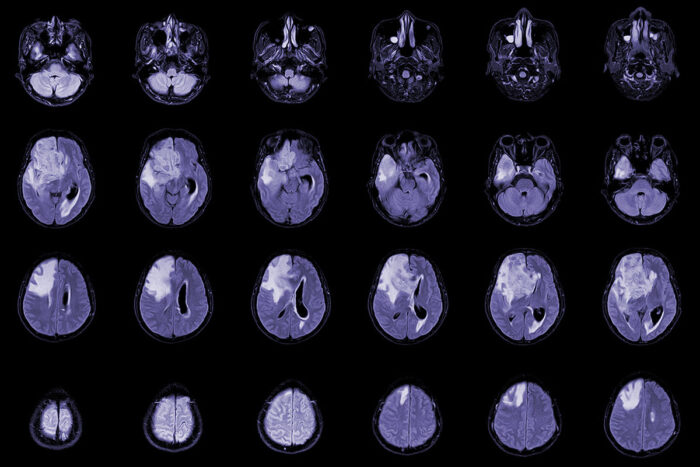
Getty ImagesA new study led by Washington University School of Medicine in St. Louis reveals at least one cause of low white blood cell counts in patients treated for glioblastoma and demonstrates a potential treatment strategy that improves survival in mice. Shown are MRI scans of a patient with this type of brain tumor.
Patients with glioblastoma, a devastating brain cancer, receive treatment that frequently leads to the unfortunate side effect of low white blood cell counts that lasts six months to a year. The low numbers of white blood cells are associated with shorter survival — but the specific reason for the prolonged drop in white blood cells and the link with shorter survival has vexed scientists.
A new study led by Washington University School of Medicine in St. Louis reveals at least one cause of low white blood cell counts in patients treated for glioblastoma and demonstrates a potential treatment strategy that improves survival in mice.
The study is published in the journal Science Translational Medicine.
Patients with this cancer typically do not survive longer than 18 months. The standard treatment is radiation and chemotherapy, after which many patients develop severely low numbers of lymphocytes — a type of white blood cell — in the bloodstream. The cause of these low lymphocyte counts has been something of a mystery because the therapy does not target the bone marrow, where these cells originate, and not all patients experience the problem.
“We know patients who develop low lymphocyte counts do worse than average,” said co-corresponding author Jiayi Huang, MD, an associate professor of radiation oncology and co-clinical director of the Brain Tumor Center at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine. “To improve their outcomes and extend their lives, we needed to understand what is causing these low levels of white blood cells and how it contributes to worse survival.”
Huang and his colleagues at Siteman led a study that collected and analyzed blood samples from glioblastoma patients. About half of the patients developed low white blood cell counts following treatment.
The researchers found a significant increase in what are called myeloid-derived suppressor cells — a type of cell known for suppressing the immune system — in those that developed low white blood cell levels. In mouse studies, the scientists then established a causal relationship between the increased myeloid-derived suppressor cells induced by irradiation of the tumor and the suppression of white blood cells.
“We wanted to find out if we could block these myeloid-derived suppressor cells using an inhibitor to improve treatment response to radiation,” said senior corresponding author Dinesh Thotala, PhD, who conducted this work at Washington University and is now with the University of Oklahoma Health Sciences Center. “We ended up testing two inhibitors separately with radiation and found a significant improvement in survival in multiple mouse models of this cancer.”
The researchers, including first author Subhajit Ghosh, PhD, a postdoctoral fellow who conducted this work in Thotala’s lab, first at Washington University and now at the University of Oklahoma, found that, on average, all of the mice with glioblastoma that received radiation alone died by day 40. Of the mice that received either inhibitor plus radiation, 50% to 60% were still alive at day 120, when the experiment ended.
One inhibitor, called CB1158, is in a class of drugs called arginase-1 inhibitors and is being investigated in a clinical trial for the treatment of solid tumors, including colorectal, lung and bladder cancers. The second drug is a phosphodiesterase-5 inhibitor called tadalafil, which is approved by the Food and Drug Administration to treat other conditions, including erectile dysfunction and pulmonary hypertension.
“The myeloid-derived suppressor cells have normal functions in the body,” said Huang, who develops clinical trials for glioblastoma patients. “During pregnancy, the body makes these cells to suppress the immune cells so it doesn’t attack the embryo or fetus. But glioblastoma takes this normal process and exploits it for its own purpose. Furthermore, radiating the tumor triggers the body to create massive quantities of these cells, leading to low white blood cell counts in our patients, sometimes for many months. Our work suggests that targeting those cells in combination with radiation may be a new, exciting strategy to improve the treatment of glioblastoma.”
To further confirm their findings, the researchers have conducted a small clinical trial that combined tadalafil with standard radiation for patients with glioblastoma. According to the researchers, they are finding encouraging results, with some patients responding well to the investigational treatment. This work is currently under review in another scientific journal. The researchers also are working toward developing new therapies with even more potential than these two inhibitors to block myeloid-derived suppressor cells.