Investigational drug attacks synovial sarcoma, a rare type of tumor
Clinical trial being planned for drug developed at School of Medicine
 Van Tine Lab
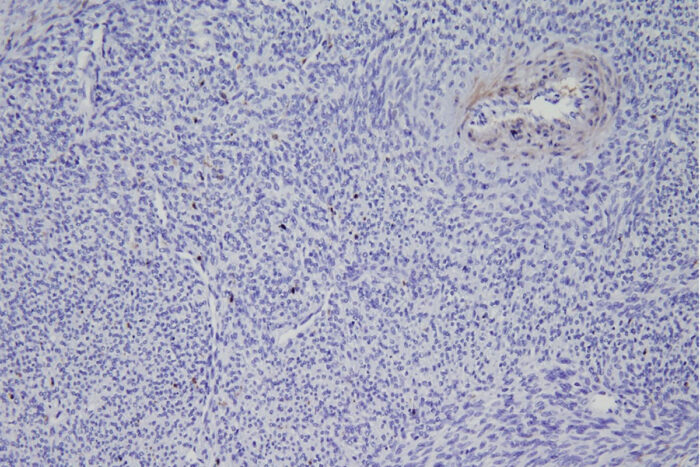
Van Tine LabA new study from Washington University School of Medicine in St. Louis describes a potential new therapy for synovial sarcoma, a rare tumor of soft tissues. Shown is a biopsy of a synovial sarcoma tumor from a patient. The staining shows that the tumor is missing an important protein called ME1. The missing protein makes this tumor type susceptible to a specific type of cell death that is triggered by a new drug being developed by a Washington University startup.
Researchers at Washington University School of Medicine in St. Louis have developed a way to attack synovial sarcoma — a rare tumor of soft tissues, such as ligaments and muscles — using an investigational drug that triggers cell death. The drug was developed by Washington University researchers who are planning a phase 1 clinical trial to investigate its safety and effectiveness in patients who have synovial sarcoma that has spread beyond the original tumor site.
The study is available online in the journal Clinical Cancer Research.
Synovial sarcoma is rare, with 900 to 1,000 new cases diagnosed annually, and is most typically diagnosed during adolescence and into young and middle adulthood. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine is a major center for the treatment of sarcomas nationwide, as is Siteman Kids at St. Louis Children’s Hospital.
“Synovial sarcoma is responsible for about 10% of all sarcomas, and because sarcoma in general is rare, a lot of these patients — pediatric and adult — travel from all over the country to receive treatment at our specialized Sarcoma Center,” said oncologist Brian A. Van Tine, MD, PhD, a professor of medicine. “If it’s diagnosed early, this cancer can be cured with standard care — surgery, radiation and chemotherapy. But once it spreads, we have no effective curative therapies, so we’re looking for new treatment strategies that take advantage of the genetic quirks of this rare tumor.”
The researchers found that synovial sarcoma is missing an important protein that most tumors rely on to drive their energy metabolism. The absence of this key protein — called malic enzyme 1 (ME1) — forces synovial sarcoma tumors to rely on a different metabolic pathway, which makes it uniquely vulnerable to the inhibition of that alternate pathway. The investigational drug ACXT-3102 interferes with this alternate route. The interference causes volatile waste compounds called reactive oxygen species to build up inside the cancer cells. When enough reactive oxygen species build up inside, the cell dies.
“Because they’re missing ME1, these tumor cells are already crippled in their ability to fight damage from reactive oxygen species,” said Van Tine, who leads the sarcoma program at Siteman. “So, we asked if we could use this broken defense against this cancer. When levels of these compounds skyrocket inside the cells, they die very quickly.”
The drug ACXT-3102 was developed by William G. Hawkins, MD, the Neidorff Family and Robert C. Packman Professor of Surgery, and his team, to treat pancreatic cancer. Because most pancreatic cancers still have ME1, researchers will need to find a second way to attack that tumor type. But because the metabolism of synovial sarcoma is unusual and consistent across patients — the cancer’s defining genetic mistake is present in 90% to 95% of all cases — the researchers suspect that this rare tumor could be treatable with ACXT-3102 alone.
“Synovial sarcoma is caused by a very specific genetic mutation, so it’s a relatively clean cancer, meaning it has a single specific genetic mistake that can be exploited, unlike other cancers that have a complex accumulation of many mutations whose effects are difficult to unravel,” Van Tine said. “Because of this single mutation, it’s harder for synovial sarcoma cells to adapt to an attack on their energy metabolism. Finding a weakness in cancer that we can exploit based on the biology of a rare tumor is really exciting.”
The drug ACXT-3102 was licensed to a Washington University startup company called Accuronix Therapeutics that was co-founded by Hawkins to develop new cancer therapies.