How does waste leave the brain?
Discovered route serves as passageway to clear fluid waste from brain
 Leon Smyth
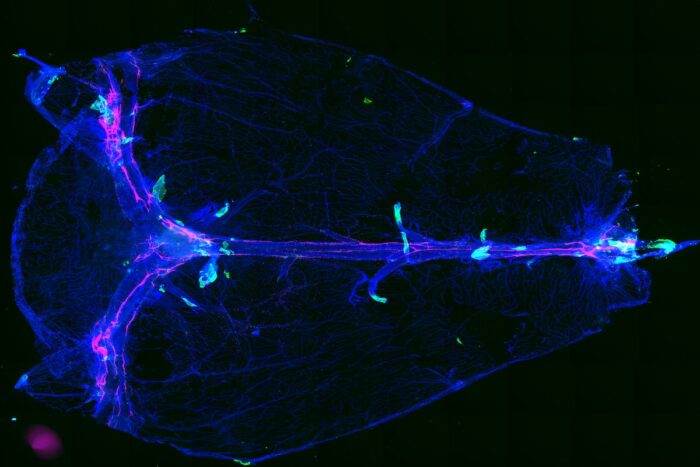
Leon Smyth Researchers at Washington University School of Medicine in St. Louis have discovered an anatomical brain structure (in green) that allows fluid waste to leave the brain. In neuroinflammatory conditions in mice, immune cells use these routes to enter the brain. The researchers think these structures and the cells and molecules positioned around them may help lead to new therapies for neuroinflammatory diseases.
Scientists at Washington University School of Medicine in St. Louis have found passageways that connect the brain to vessels that carry fluid waste out of and away from the brain. The newly discovered anatomical structures, found in mice and people, are like tiny gates, allowing waste to leave the brain and enter lymphatic vessels, where immune cells monitor it for signs of danger or infection.
These structures can be likened to airport security checkpoints. Molecules and fluid move in both directions through the gates, but immune cells are tightly regulated. Under normal conditions, immune cells are not allowed past security — similar to sharp objects, firearms and other potential weapons in an airport.
“Any opening can become a weak point when safeguards fail,” explained Jonathan Kipnis, PhD, the Alan A. and Edith L. Wolff Distinguished Professor of Pathology & Immunology and a BJC Investigator. “We have identified a previously unknown route that immune cells can use to access the brain in diseases driven by inflammation. We think these structures, and the cells and molecules strategically positioned around the gates to control passage, can help lead to new drugs for neuroinflammatory diseases.”
The findings are available Feb. 7 in Nature.
Immune cells and molecules play an important role in normal brain development and function, and in neurological conditions with an inflammatory component, such as Alzheimer’s disease, multiple sclerosis and Parkinson’s disease.
“The immune system communicates with the brain using molecules that cross from the dura mater to the brain,” said Kipnis, who in 2015 discovered the presence of lymph vessels in the dura mater, the outer tissue layer enveloping the brain underneath the skull. “But miscommunication between the brain and the immune system can have detrimental effects on brain function, including memory and behavior.”
When gatekeeping fails, immune cells can infiltrate the brain inappropriately and trigger inflammation. In mice with multiple sclerosis – a chronic neuroinflammatory disease in which the immune system attacks the central nervous system – the researchers saw immune cells traveling through the gates as part of this study. They did not see the cells migrating in healthy mice, because other cells and molecules stop the immune cells from crossing.
The discovery of the gates also resolves a physiological mystery. Immune cells stationed in the dura mater monitor brain fluid for signs of injury or infection as it flows out of the brain. This immune surveillance is critical for maintaining brain health. But sandwiched between brain and the dura mater is the arachnoid barrier, which physically hinders movement of molecules, fluid and cells. So how is the fluid getting out?
“It’s a paradox. On the one hand, the barrier is impermeable and does not allow anything to get out of the brain,” said Leon Smyth, PhD, a postdoctoral researcher in the Department of Pathology & Immunology and the study’s first author. “On the other hand, cerebrospinal fluid must get through the arachnoid barrier, because we can detect it on the other side.”
To understand how fluid passes through the arachnoid barrier to reach the dura mater, the scientists injected mice with light-emitting molecules. They found that fluid slips through these gates in the arachnoid barrier near blood vessels that pass through it.
MRI imaging of healthy people revealed that similar gates also are found in the human brain. Kipnis collaborated with Danny S. Reich, MD, PhD, a senior investigator at the National Institute of Neurological Disorders & Stroke of the National Institutes of Health (NIH), to capture injected dye flowing through gate-like structures in people’s brains.
Now the researchers are looking deeper into other diseases in which neuroinflammation plays a role in disease progression, including Alzheimer’s disease, in which impaired waste clearance leads disease-causing proteins to build up in the brain.
“If a drain is clogged, wastewater will back up in the sink,” Kipnis explained. “The clog in the drain needs to be addressed so the water can go through the piping. We think the same may be true in the brain. A clog in these gates may prevent waste from leaving the brain in neurodegenerative disease. Perhaps if we can clean the clogged gates, we can protect the brain.”






