Device for noninvasive brain biopsies via blood draw moves closer to market approval
FDA grants WashU-based technology ‘Breakthrough Device’ designation
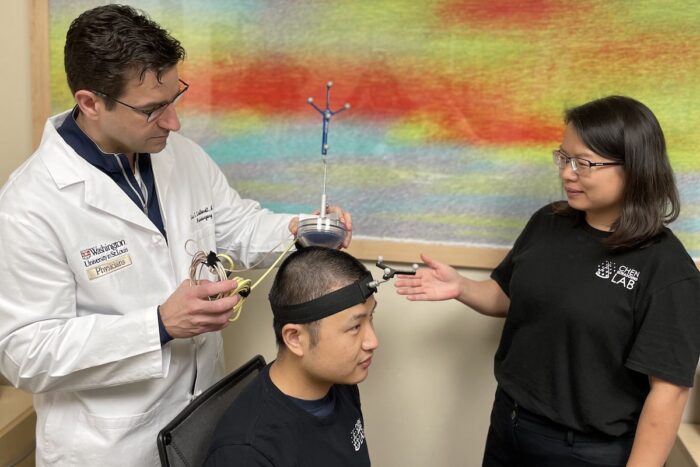 Courtesy of Hong Chen
Courtesy of Hong ChenGraduate student Lu Xu (center) wears a device co-invented by Washington University’s Eric C. Leuthardt, MD, (left) a professor of neurosurgery at the School of Medicine, and by Hong Chen, PhD, (right) an associate professor of biomedical engineering at the university's McKelvey School of Engineering. The technology was developed so that noninvasive biopsies could be performed in adults with brain tumors. It has been granted U.S. Food and Drug Administration "Breakthrough Device" designation, which helps accelerate the process toward market approval.
A device aimed at enabling neurosurgeons and other physicians to perform noninvasive blood-based biopsies in adults with brain tumors has received Food and Drug Administration (FDA) “Breakthrough Device” designation. The device includes technology from Washington University in St. Louis and developed by Cordance Medical Inc., a medical device company in Mountain View, Calif.
The designation is aimed at providing patients and health-care providers with timely access to novel medical devices by expediting the review process needed to bring technology toward market approval.
The FDA distinction applies to devices that have undergone rigorous review and show exceptional promise of improved treatment or the ability to diagnose life-threatening or debilitating diseases. The technology was co-invented by Eric C. Leuthardt, MD, the Shi Hui Huang Professor of Neurosurgery at Washington University School of Medicine, and by Hong Chen, PhD, an associate professor of biomedical engineering at the university’s McKelvey School of Engineering and of neurosurgery at the School of Medicine.
Washington University recently licensed the technology to Cordance Medical. Leuthardt and Chen serve as advisers and shareholders of Cordance Medical, which is involved in commercializing the technology.
The device — called NeuroAccess — is portable and noninvasive, and it allows physicians to avoid high-risk brain surgery to obtain blood-based liquid biopsies of patients who have known or suspected brain tumors. The device uses sonobiopsy, a technique that targets the blood-brain barrier, a protective, semi-permeable membrane that prevents harmful substances in the blood from contaminating sensitive tissue in the brain. The interaction of ultrasound and microbubbles causes the blood-brain barrier to open temporarily so RNA, DNA and proteins from the brain can diffuse into the blood.
“The sonobiopsy approach is a revolution in brain diagnostics,” said Leuthardt, also a professor of biomedical engineering, of mechanical engineering and of neuroscience. “Just as magnetic resonance imaging (MRI) transformed how we look at the anatomy of the human brain, sonobiopsy will transform how we examine the brain on a molecular level. Essentially, we’re doing a brain biopsy without the high risks of brain surgery.”
The researchers published a study in September, in the journal NPJ Precision Oncology, that found sonobiopsy was feasible and safe for use in people.
“The breakthrough designation is the success of a team that spans numerous disciplines ranging from medicine, engineering, genetics, neuroscience and entrepreneurship to bring this concept from a very basic scientific model to an ongoing clinical trial,” Leuthardt said. “This is a triumph of collaboration.”
Said Chen, also a professor of radiation oncology and of radiology: “Sonobiopsy is poised to open doors to diagnose brain diseases in which surgical biopsies are often not an option. The technology presents new opportunities in the diagnosis of neurodevelopmental, neurodegenerative and psychiatric disorders.”
Leuthardt is the director and Chen is a member of the Division of Neurotechnology in the Department of Neurosurgery, which focuses on multidisciplinary research to create innovative engineered solutions that can be translated to patients with neurologic diseases. Washington University owns a patent on the sonobiopsy technology.