Researchers solve medical mystery of deadly illness in young child
Cross-disciplinary team identifies genetic cause of rare, undiagnosed lung disease
 Jiehong Pan
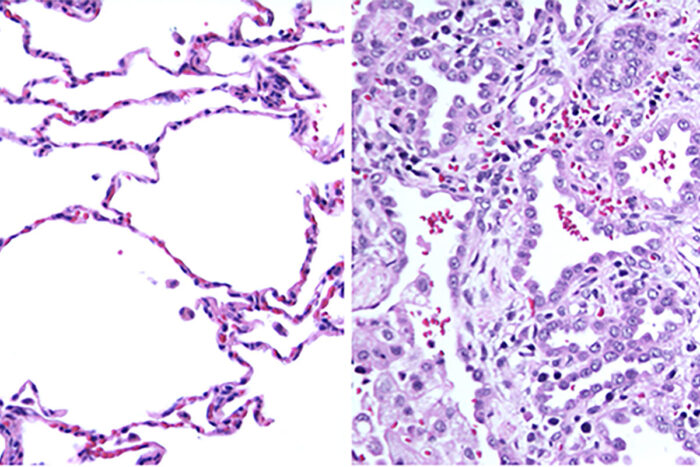
Jiehong PanNew research from Washington University School of Medicine in St. Louis has solved the medical mystery of why a 2-year-old child — seemingly healthy at birth — succumbed to an undiagnosed, rare illness. On the left is normal lung tissue showing air sacs with thin cell layers for the exchange of oxygen and carbon dioxide. On the right is the patient's lung tissue. Because of a mutation in the RAB5B gene, the walls of the air sacs are thick and unable to participate in gas transfer.
New research from Washington University School of Medicine in St. Louis has solved the medical mystery of why a 2-year-old child — seemingly healthy at birth — succumbed to an undiagnosed, rare illness. The research team identified a previously unknown genetic cause of interstitial lung disease, providing answers to the parents and doctors puzzled by the child’s condition.
The research, conducted as part of the National Institutes of Health’s (NIH) Undiagnosed Diseases Network, demonstrates, among other benefits, how an interdisciplinary team of researchers can work together to solve medical mysteries, helping patients understand a diagnosis, prognosis and what a genetic abnormality may mean for family members and family planning.
The study is published the week of Jan. 31 in the Proceedings of the National Academy of Sciences. The Undiagnosed Diseases Network is a national research network aimed at diagnosing rare and previously undescribed diseases in patients whose conditions present as medical mysteries. Washington University serves as a clinical site that evaluates patients, and a model organism screening site that develops models to study genes in zebrafish and roundworms.
Interstitial lung disease is a broad term for a disease in which the lungs gradually deteriorate, causing scarring that makes it increasingly difficult to breathe. Several gene abnormalities have been associated with interstitial lung disease in infants and children, but some patients have the disease despite harboring none of the known genetic abnormalities. In the new study, the researchers were presented with the case of a young child with interstitial lung disease of unknown cause. The child later died of the disease.
The researchers analyzed the child’s DNA code as well as the DNA code of both parents. A team of bioinformatics specialists at Baylor College of Medicine then narrowed down the initial long list of DNA code changes or genetic variants they identified — many of which are harmless — to a smaller list of possible culprits. The lung tissue from the child had evidence of a problem with surfactant in the lungs. In the lungs’ air sacs, surfactant is a complex mixture of proteins and lipids that reduces surface tension in the air sacs and keeps them open, easing the exchange of oxygen and carbon dioxide during breathing. Many people with interstitial lung disease have abnormalities in the surfactant protein genes. But this child did not have any genetic variants in the code of the surfactant protein genes.
Rather, the researchers found a variant in a gene that makes a protein called RAB5B that turns out to be part of the cellular machinery that processes the surfactant proteins, the researchers later learned. They showed that the RAB5B protein plays a vital role in packaging the surfactants into tiny compartments called vesicles and moving them to their proper locations. In this case, the genetic variant did not simply prevent the protein from working — the genetic variant caused the protein to be actively harmful.
“When mutations happen that break a protein, usually the protein just doesn’t work anymore — its function is missing,” said co-senior author Tim Schedl, PhD, a professor of genetics. “But this is a case where the broken protein is not only not working, it’s actively poisoning other processes. This results in the loss of the surfactants in the lungs.”
The researchers were able to identify this abnormality by studying the genetic variant in roundworms that are called C. elegans. The child had only one abnormal copy of the gene, demonstrating that even having one normal copy did not compensate for the poisonous protein produced by the mutated copy. Worms with one abnormal copy required three normal copies to restore normal function, demonstrating the poisonous activity of the abnormal copy, according to experiments conducted by first author Huiyan “Winnie” Huang, PhD, an instructor in pediatrics. And consistent with these genetics, the researchers found that neither of the child’s parents had the genetic abnormality, indicating that the variant was only present, by happenstance, in the child’s genes and was therefore a new variant in the DNA that arose during embryonic development.
“In so many cases, we don’t know why a patient has a particular disease,” said co-senior author Steven L. Brody, MD, the Dorothy R. and Hubert C. Moog Professor of Pulmonary Medicine. “But we were able to solve this case, and there’s a real satisfaction in that. Potentially, this could lead to finding answers for other people who have diseases similar to this.”
Added co-author Jennifer A. Wambach, MD, an associate professor of pediatrics: “This gene, RAB5B, is now associated with interstitial lung disease in children. There are patients with a clinical diagnosis of interstitial lung disease without a genetic explanation. For these patients, sequencing RAB5B may reveal changes in their DNA code that could account for their disease. Knowing the underlying genetic cause and identifying other patients with the same genetic problem can help us better predict the course of the disease, so we can better prepare patients and their families for what is to come, such as whether the patient may respond to treatments, or worsen to needing a lung transplant, or whether it may be appropriate to begin discussing compassionate care.”
While the diagnosis was not able to help the patient in this case, knowledge of the underlying cause allowed the parents to know that the genetic variant was not inherited and there would be a very low chance of future children having the same disease.
“Because these types of genetic diseases are so rare, there’s very little information out there for patients or families,” said co-senior author Stephen C. Pak, PhD, an associate professor of pediatrics. “But collectively, there are millions of people who live with rare genetic diseases. That’s why the Undiagnosed Diseases Network was formed — to bring together bioinformatics specialists, researchers, lung biologists, pediatricians and other experts into this type of unique collaboration to try and address this unmet need.”