Podcast: Can boosting the immune system, rather than suppressing it, work against COVID-19?
This episode of ‘Show Me the Science' details research findings that patients with COVID-19 often develop weakened rather than hyperactive immunity in response to the coronavirus
 Matt Miller
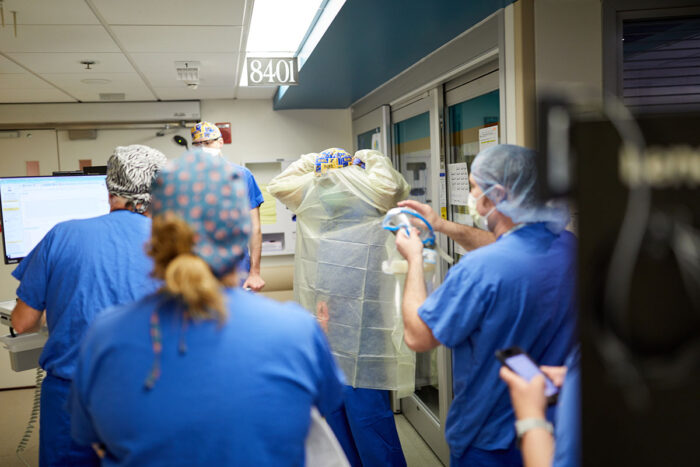
Matt MillerMedical staff in an intensive care unit at Barnes-Jewish Hospital get into their protective gear before entering the room of a patient with COVID-19. New research from scientists at Washington University School of Medicine in St. Louis suggests that the immune systems of such patients can’t do enough to protect them from the virus. The researchers are proposing boosting the activity of immune cells to treat some patients with COVID-19.
A new episode of our podcast, “Show Me the Science,” has been posted. At present, these podcast episodes are highlighting research and patient care on the Washington University Medical Campus as our scientists and clinicians confront the COVID-19 pandemic.
New research from scientists at Washington University School of Medicine in St. Louis suggests that the immune systems of COVID-19 patients can’t do enough to protect them from the virus.
A popular theory has it that patients’ immune systems get so revved up fighting the virus that, after several days, a so-called cytokine storm ensues, resulting in potentially fatal organ damage, particularly to the lungs. But new findings from a team of researchers led by Richard S. Hotchkiss, MD, a professor of anesthesiology, and Kenneth E. Remy, MD, an assistant professor of pediatrics, have found that many patients get very sick because their immune systems can’t do enough to protect them from the virus. They’re suggesting that rather than trying to dampen the immune response, a better treatment strategy for COVID-19 would involve boosting immunity.
The podcast, “Show Me the Science,” is produced by the Office of Medical Public Affairs at Washington University School of Medicine in St. Louis.
 Related: Boosting immune system a potential treatment strategy for COVID-19
Related: Boosting immune system a potential treatment strategy for COVID-19
Researchers find weakened, rather than hyperactive, immunity in response to virus
Transcript
[music plays]
Jim Dryden (host): Hello and welcome to Show me the Science, a podcast about the research, teaching and patient care, as well as the students, staff and faculty at Washington University School of Medicine in St. Louis, Missouri – the Show Me state. My name is Jim Dryden and I’m your host this week. We’ve been focusing these podcast episodes on the COVID-19 pandemic and Washington University’s response. This week, the immune systems of COVID-19 patients and some of the ways they may be similar to patients who have sepsis, a potentially life-threatening condition that’s caused by the body’s response to an infection. Sepsis occurs when the body releases immune molecules into the bloodstream to fight the infection, but the body’s response gets messed up. When the COVID-19 pandemic began, we heard many stories about how people would be sick for a few days but then later, even after some had started to feel better, they would have to be hospitalized, often fighting for their lives. The conventional wisdom was that patients’ immune systems got so revved up fighting the virus that they also caused collateral damage to the patient’s own tissues in what scientists called a cytokine storm, a process similar to what happens in some sepsis patients. But Washington University intensive care unit specialist Richard Hotchkiss thinks, in many cases, that’s not what’s happening. Hotchkiss is an expert on sepsis who has been arguing for years that sepsis often is not a cytokine storm disease, but rather is an immunosuppressive disease. Looking at patients with COVID-19, he sees a number of similarities.
Richard Hotchkiss, MD: People are treating COVID with diametrically opposed drugs. Everybody’s throwing the kitchen sink at it, and predominantly early on, it was to block the immune system. It may be true that some people do die from a hyper inflammatory, or do poorly. But it looks like that if you block the immune system too much, you’re not going to be able to control the virus.
Dryden: Hotchkiss, whose prior work led to changes in treatment for some patients with sepsis, and fellow critical care specialist Ken Remy, used a technique called ELISpot to look at the immune cells in the blood of COVID patients who were hospitalized at several hospitals in St. Louis, including Barnes-Jewish Hospital and Missouri Baptist Hospital, or MoBap. They recently published their findings in the scientific journal JCI Insight. Remy says what they found was that many sick patients had a weakened immune response rather than a cytokine storm. And they also found that a drug they’ve used to treat some patients with sepsis, called interleukin 7 or IL-7, might also help in some COVID patients.
Kenneth Remy, MD: Your immune system, when activated by an infection or some sort of trauma, has a number of different types of cells, if you will, the sort of reactive cells that will release certain little proteins. And some of these proteins are called chemokines. They bring other cells to the party. And then other cells release cytokines. And the way that I think about a hyper- or pro-inflammatory state is that the immune system, now, is working on overdrive. It’s super-inflamed, and the storm is a release of these proteins, these cytokines, which subsequently causes this overwhelming inflammation. And so this phenomenon of cytokine storm was really released, I think, early by folks maybe in China and Italy. And everyone sort of latched on to that as if it were exact science, and it became the dogma for what the disease entity became, at least in March and April. Now, Dr. Hotchkiss has been working for a number of years, certainly, in understanding the immune system in inflammatory diseases such as sepsis. And so it was really interesting, because we had good dialogue discussing this disease, and wanted to further evaluate the immune system, because it just didn’t sound right to us.
Hotchkiss: So what’s so amazing about the COVID is that people are treating COVID with diametrically opposed drugs. For example, there was one study using a drug called GM-CSF, and another study from another group giving a drug with that blocked GM-CSF. I mean, it’s incredible. Everybody’s throwing the kitchen sink at it. And predominantly, early on, it was to block the immune system. It may be true that some people do die from a hyper inflammatory or do poorly. But it looks like that if you block the immune system too much, you’re not going to be able to control the virus. And in autopsy studies, there’s a virus present in a lot of the– there’s evidence that’s present a lot of the organs of people that die, suggesting that your immune system is not working well.
Remy: Without ever looking at a tire, if you were told that the tire was overinflated and potentially could pop, and you never looked at it, but you then assumed, “Well, to fix this, what we’re going to need to do is go out there and poke holes into the tire, let some air out.” But when you actually go and look at it and evaluate it and examine it, and you notice that the tires actually were quite under-inflated, and now you’re still going to go out there and poke holes in it, you’re now going to worsen the under-inflation and the tire is never going to function.
Dryden: The patients who had COVID-19, they actually had fewer cells that make these inflammatory molecules called cytokines. But the cells that they did have also were not as efficient at secreting those cytokines. So they have a double whammy: fewer cells making this stuff, and the stuff they’re making isn’t as good as that. Is that correct?
Remy: Correct. Which then leads for potential for unrivaled or unmitigated replication of the virus, and the ability for secondary infections to come in and actually cause additional hits, if you will, to their underlying disease.
Dryden: You compared the patients who had COVID-19 to patients who had sepsis, and to critically ill patients who didn’t have either of those disorders. Is the comparison between COVID-19 patients and sepsis patients a little closer, maybe, than was thought?
Hotchkiss: I would say yes. There is immune suppression in both septic patients and COVID patients. We think that, from sepsis, from all the studies that we’ve done in the past, the longer the sepsis persists, the more likely the patients are to develop this immune suppressive phenotype. They get T cell exhaustion. The patients that predominantly die from COVID are elderly patients. I mean, they’re people that have compromised immune systems because of immunosenescence. You know, as you get older, there’s this immunosenescence. Your immune system isn’t as functional. And so it didn’t, theoretically, make good sense that a lot of these patients were dying from hypercytokinemia. And the opposite: Why are the people that are dying the ones that have the compromised immune systems? Patients with COVID develop very low circulating lymphocyte counts, which correlates with mortality.
Dryden: And so you want to boost their cytokines with IL-7. And there is a clinical trial going on right now in Great Britain, correct?
Hotchkiss: In the UK, they’ve treated seven patients. And we hope to begin it here. We’re waiting for FDA approval here in the US.
Dryden: You have a long history with IL-7 as a researcher. When you talk about these patients with low lymphocyte counts, I mean, I’m reminded of AIDS patients in the ’80s and ’90s.
Hotchkiss: So, Jim, you’re very prescient on that. They were doing a trial– NIAID was very excited about it, too. In fact, [Anthony] Fauci [the director of the National Institute of Allergy and Infectious Diseases] did some work on IL-7. It did restore the T cell count safely in HIV patients. The problem was – not a problem, a great thing. The antiretroviral therapy and the protease inhibitors came out. And basically, if you stay on those medications, you’re going to do fine, if you stay on them. You didn’t need IL-7 anymore. It has been lifesaving in some other viral infections. It’s been used in other things. And it’s in active clinical trials now in oncology to boost the immune system. We think it’s kind of a good immunotherapy.
Remy: When used in the appropriate population.
Hotchkiss: Absolutely.
Remy: And that’s the key thing is that, the work that we just published, it really is about defining the population. And I’m happy that us and other groups have really had a paradigm shift, at least in this disease, by demonstrating the data that we have. And that’s why things like IL-7, when applied to the right patient population, will have success.
Dryden: Who are the right patients, though? Is it the patient that is sick enough to be hospitalized but not yet in the ICU? Is it the patient that’s in the ICU but not yet ventilated?
Remy: That’s a great question, because it really hinges on a couple of things. If we were to get a population across different severities of illness – this paper was looking at really sick patients, and we’re now evaluating some different patients now – we would be able to really define, I think, the patients that would benefit from these immuno-restorative medications based off of what we see in the dish. So I think you’re highlighting a need for us to be able to pair a functional readout against the clinical severity of illness, but also be able to evaluate a drug in a dish, and then put it into a patient to see for success.
Hotchkiss: This COVID pandemic underscored the need for a way to figure out the immune status on these patients, because they’re throwing drugs, a lot of drugs, that could do potential harm. They’re assuming it’s hyperinflammatory. They’re testing drugs that have exact opposite effects in the same disease. And they don’t have a good way to identify whether the patient is in the hyperinflammatory or the immune suppressed state, and they don’t have a good way to follow their therapy. We really think that the ELISpot assay, because it’s a great functional assay, will help in this.
Remy: There really is this major need to be able to adopt therapies directed at some sort of objective marker.
Hotchkiss: The predominant thinking was that it was hyperinflamed. The work that we did, and the work that many of our team members did– I can’t emphasize enough about the team thing.
Dryden: There were a lot of people involved in this effort, both here in St. Louis and around the world.
Hotchkiss: Exactly. And I have to give credit. Ken, personally, was in the COVID units at MoBap all the time, and here at WashU. I was kind of hesitant to do the blood studies at first. And so he said, “No, we’ve got to do it.” He pushed it. He drove this thing about getting the patients, and personally went there, was in the units all the time, got the guys at MoBap excited. MoBap, I mean, they made a major contribution. Ken was driving all that. And our lab people, I mean, they were in here every day, sometimes on Saturdays, doing these COVID– we have the best lab. I’m blessed with an amazing group of people, and with a group at the University of Florida, Gainesville. I mean, it was just unbelievable.
Remy: We just had everybody, either in person operating at the lab, or at home remotely gathering data. And every time I would work overnight in a COVID unit at BJH and drive over to MoBap, and we would have a system where I would go to MoBap, and sometimes I would call Richard while driving back, saying, “I am so tired. Can someone meet me in the circle to pick up these samples from my car?” And Dale Osborne, who is a superstar–
Hotchkiss: He did!
Remy: –would run outside and get them, and then give them to Ali [Ellebedy], who was waiting at the ward for his piece of the pie, if you will. I mean, everyone was just–
Hotchkiss: I don’t know how Ken’s still alive after this. I’m surprised he’s still married, too.
Remy: We worked hard together.
Hotchkiss: Yeah. Well, you worked the hardest, I think.
Remy: In fact, that’s probably the best thing. We talked about how we saw it, that there are a number of groups that were wrong with cytokine-storm belief, but a lot of those folks are publishing this stuff in isolation, only out of a single lab. What you’ll notice from our paper, you’re looking at multiple groups all working together. And that’s nice, because you’ve got your own internal peer review.
Dryden: A few minutes ago when I tried to make the comparison to the immune profile of an HIV patient, and you said, fortunately we don’t have to use IL-7 in those patients, because they’re able to take other medications, and they never get to that point. Is there anything on the horizon that either of you see in terms of vaccine, monoclonal antibodies, other treatments that might keep patients from getting to that point where they would need this drug?
Hotchkiss: Yeah, that’s a great– yes. The antibodies are going to work. It’s going to be– I predict it’s going to be gone. I mean, because you’re going to have these antibodies that work, and the vaccines. But what is going to come is other pandemics. They should have been looking for this! They should have been already geared up! What is going to happen, and what we’re working on now, Ken and I are– this is the thing about IL-7. The immune system is the bottom line. If you can boost your immune system, you’re going to make people more resistant to any of the pandemics that are, coming or more able to survive. The focus of infectious disease should shift now somewhat to the host. That is the central point that should come from some of this.
Remy: I disagree with him. I don’t think COVID’s going to go completely away. I think we’re going to have some COVID disease. Certainly, we’re going to use neutralizing antibodies to try to mitigate some of the severity upfront. But the problem is is that patients– their immune systems are going to already have endured an insult to some degree. And so it’s not unreasonable to believe that they’re going to have potentially some suppression of the immune system in time, which will render them susceptible, perhaps, to other infections, even COVID as the initiating virus. If you think that way, then when we get to the fall, when we get to human metapneumovirus and RSV, and we get to influenza season and a whole host of other things, there’s reason to believe that people could certainly get infected with those diseases. And if they had restoration of their immune system so that they’re able to successfully fight these diseases, even after the insult of COVID, then in fact they may have a better outcome. And so IL-7 could be helpful there from that perspective. The other area that IL-7 could be helpful is not as a standalone drug, but in combination with other things, including vaccine or some of these other therapies, because that may prolong the effects. By stimulating a person’s immune system, they may actually have a better efficacy after a vaccine, or after some of the other therapies. There may be combination therapies that also may have quite good efficacy for this disease, and/or if we’re faced with other pandemics.
[music plays]
Dryden: As they work to try to do better with this pandemic, Remy and Hotchkiss hope to begin clinical trials using IL-7 to stimulate the immune systems of COVID patients whose blood tests, perhaps conducted with something like ELISpot, show that their immune cells have been suppressed. They’re also working on strategies that, in the long run, might make some of us less susceptible to problems the next time that a viral pathogen decides it’s about time to turn the world upside down.
Show Me the Science is a production of the Office of Medical Public Affairs at Washington University School of Medicine in St. Louis. The goal of this project is to keep you informed, and maybe teach you some things that will give you hope. Thanks for tuning in. I’m Jim Dryden. Stay safe.
[music plays]






